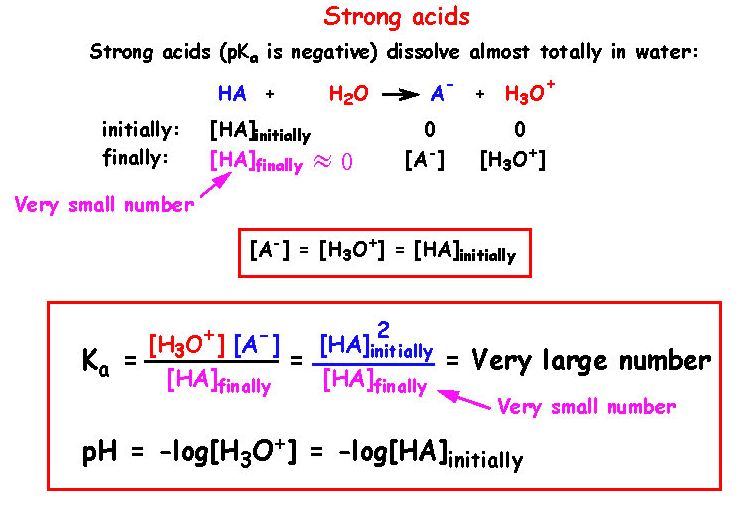
A 0.21 M solution of chloroacetic acid, ClCH2CO2H, has a pH of 1.79. Calculate Ka for the acid. | Homework.Study.com
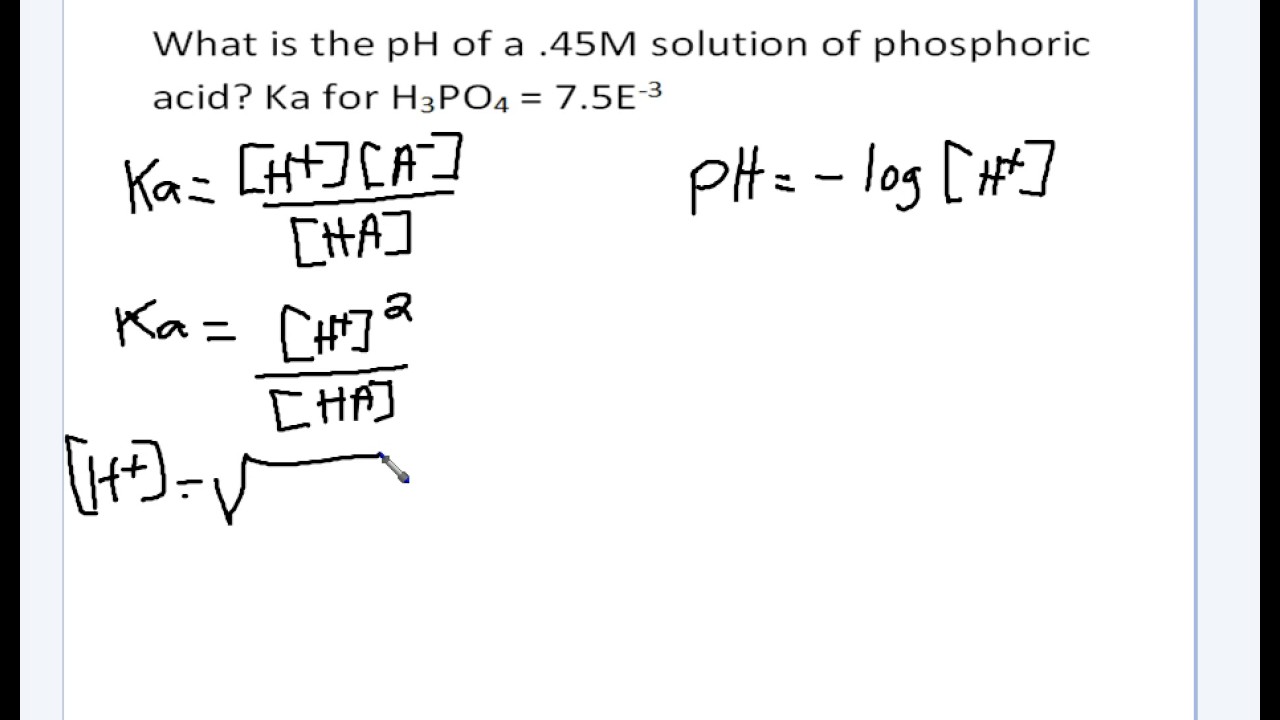
![Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/161343719_web.png)
Calculate Ka of acetic acid from equilibrium concentration given below: [H(3)O^(+)]=[CH(3)COO^(-)]=1.34xx10^(-3)M, [CH(3)COOH]=9.866xx10^(-2)M
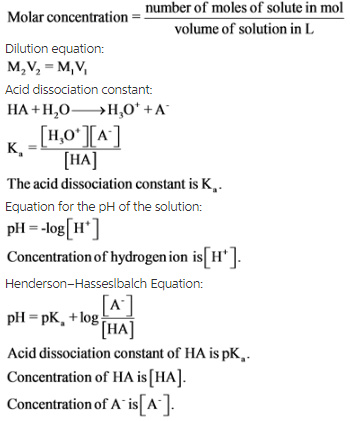

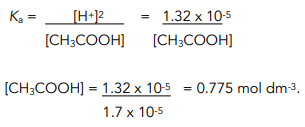



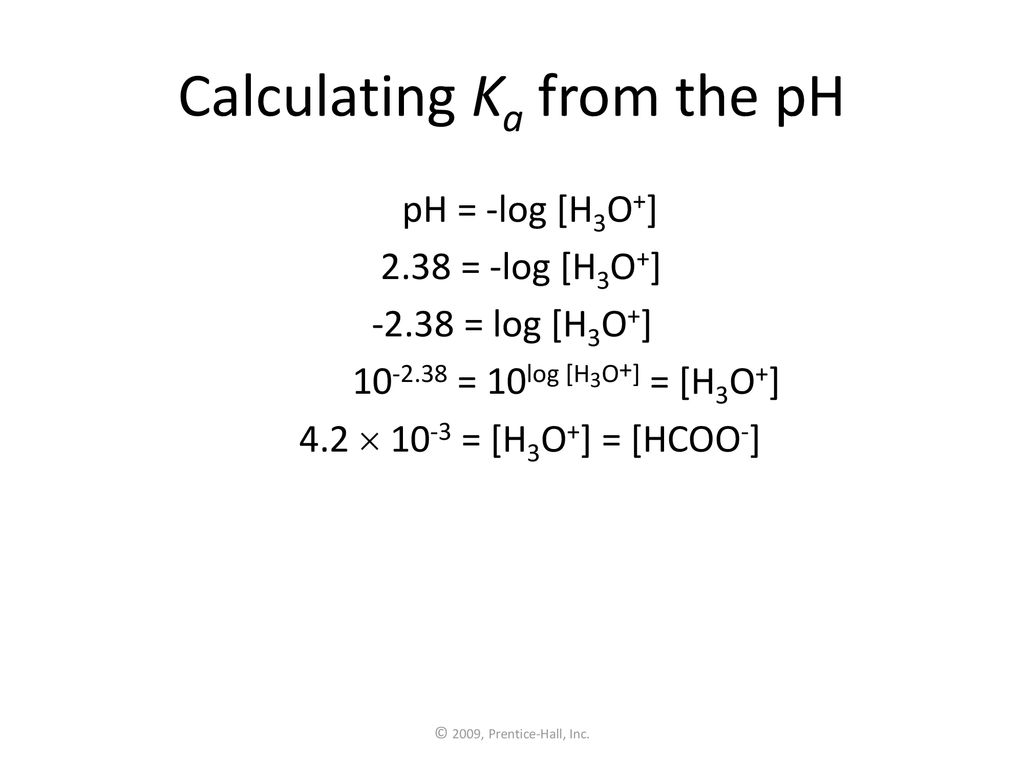



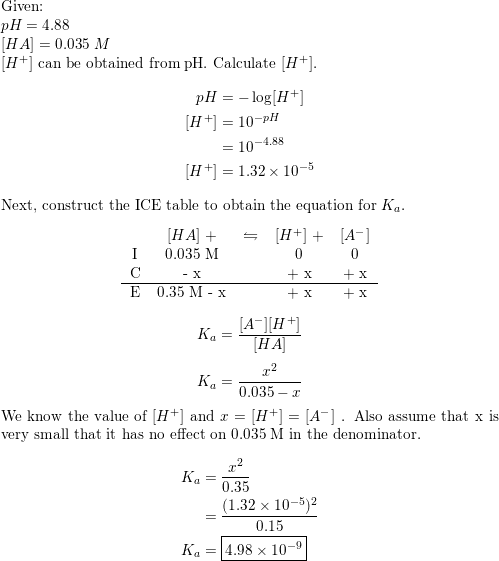
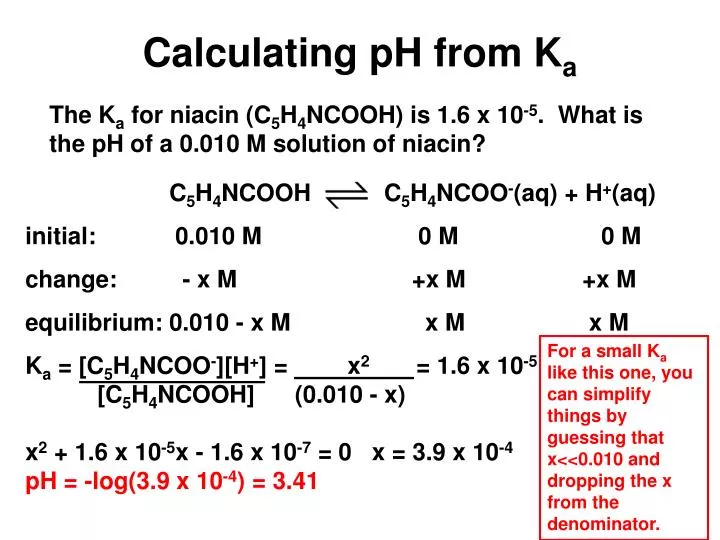
![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)